VIPUN Medical announces the publication of the ANTERO-1 clinical study, evaluating the ability of the VIPUN Gastric Monitoring System to distinguish normal versus pharmacologically- impaired gastric motility, in the Journal of Neurogastroenterology and Motility.
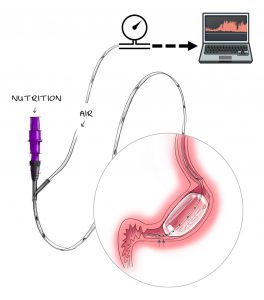 VIPUN Medical announces the online publication in the Journal of Neurogastroenterology and Motility of the results from the completed First- In-Man clinical study, ANTERO-1, studying the safety, feasibility and functionality of the VIPUN Gastric Monitoring System (VIPUN GMS) in healthy subjects.
VIPUN Medical announces the online publication in the Journal of Neurogastroenterology and Motility of the results from the completed First- In-Man clinical study, ANTERO-1, studying the safety, feasibility and functionality of the VIPUN Gastric Monitoring System (VIPUN GMS) in healthy subjects.
“We are very pleased with the publication of the ANTERO-1 study in a relevant, peer-reviewed medical journal.“ said Pieter Janssen, Chief Technology Officer of VIPUN Medical. “The results demonstrate the potential of the VIPUN GMS to diagnose opioid-induced impaired gastric motility. Moreover it shows a favorable safety profile and remarkable ease of use.”
The publication can be accessed here: https://onlinelibrary.wiley.com/doi/full/10.1111/nmo.13733
About ANTERO-1
ANTERO-1 is a randomized, single‐blinded, cross‐over study conducted in the University Hospital Gasthuisberg in Leuven, Belgium. In this study, healthy volunteers received either placebo or codeine while motility‐induced pressure changes were recorded using the VIPUNG GMS for 6 hours, including a period of 2 hours while nutrients were infused. An algorithm, designed to detect phasic contractility, converted pressure changes to a gastric balloon motility index which was correlated to the emptying speed of the nutrients as determined with the 13C- octanoate breath test.
About VIPUN Medical
VIPUN Medical, a KU Leuven spin-off is a development stage, privately held, medtech company aiming to improve clinical outcomes in critically ill patients. VIPUN Medical’s development stage product, the VIPUN GMS, is in-licensed from the University of Leuven, Belgium.





