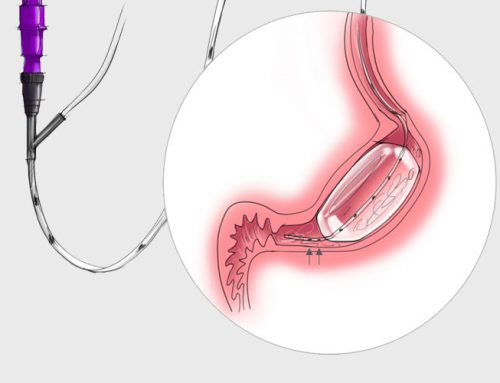
VIPUN Medical announces topline study results from LOTUS clinical investigation performed to validate the VIPUN Gastric Monitoring System (GMS) as a gastric motility monitoring system
- Data show a strong correlation in the duration of gastric contractility measured by means of the VIPUN GMS and manometry (golden standard) technique.
- Data show that the contraction frequency automatically detected by the VIPUN GMS, is strongly correlated with that observed by expert gastroenterologists on manometry recordings.
- Data show that the VIPUN GMS can be used to identify hallmarks of gastrointestinal motility, such as the MMC cycle and postprandial accommodation reflex.
Mechelen, Belgium, June 5, 2023 – VIPUN Medical, a company engaged in the development of innovate nutrition devices today announced positive clinical study results from LOTUS, a prospective, interventional, single-arm, monocentric, investigation in healthy adult humans designed to validate the VIPUN GMS as a gastric motility monitoring system.
The primary hypothesis, that the duration of gastric contractility measured by means of the VIPUN GMS and manometry (golden standard technique) are strongly correlated, was confirmed, both at the level of 10-min periods (r = 0.92, p < 0.001) and at subject level (ρ = 0.96, p < 0.001). A strong correlation was defined up front as a coefficient ≥ 0.7.
The secondary hypothesis, that the contraction frequency observed by expert gastro-enterologists on manometry is strongly correlated with the contraction frequency automatically detected by the VIPUN GMS, was confirmed, both the level of 10-min periods (r = 0.68, p < 0.0001) and at subject-level (ρ = 0.87, p = 0.001).
Expert gastroenterologists agreed on the presence/absence of hallmarks of normal gastric motility on both technologies. The intra-reader agreement was on average 84.6% for the identification of a MMC cycle and 76.2% for the identification of the accommodation reflex. These results indicate that both technologies allow a similar interpretation.

Overall, the results are in line with the investigation hypotheses and support the conclusion that this direct comparison versus the gold standard provides solid evidence on the performance of the VIPUN GMS as a gastric monitoring system. The LOTUS Investigation was performed at the Translational Research Center for Gastro-intestinal Disorders (TARGID) in the University Hospital Leuven led by Prof. Dr. Jan Tack.
‘The investigation is a part of the clinical program evaluating the safety and performance of the VIPUN Gastric Monitoring System. These results underline the potential of the VIPUN GMS to significantly change the way clinicians monitor gastric motility and provide enteral nutrition.’ said Pieter Janssen, VIPUN Medical CTO. ‘We look forward to execute our further plans for clinical validation of the VIPUN GMS, and offer the potential to directly enhance care, especially in critically ill patients.’
About VIPUN Medical
VIPUN Medical is a privately-owned clinical stage medical device company, pioneering a novel method of gastro-intestinal monitoring aimed at enhancing medical nutrition for critically ill and other vulnerable patients. The VIPUN Gastric Monitoring System aims to make it easy for medical staff to make a well-informed and faster nutrition therapy decision thus reducing malnutrition and feeding-related complications. VIPUN Medical is located in Mechelen, Belgium.
VIPUN Medical Media Contact
Nico Van Tichelen, +32 495 67 40 47
nico.vantichelen@vipunmedical.com